Mines and ores
Some layers of this laterite were rich in iron oxides and hydroxides and were exploited as ores. Generally, these are deep strata and minders dug pits 5-10 meters down to reach them (fig. 1).
Clayey sediments from the Paleozoic covering layers rich in iron are found at the base of the Bandiagara escarpment. In several places, they were exploited by mine pits.

Fig.2 Great slag mass, showing that numerous reduction operations were performed at Tinntam. Photo V. Serneels
Reduction of iron ores
In the ores, iron is found in the form of oxides that are mixed with various other substances (sand, clay, etc.). To produce metallic iron, one has to:
1. Cause the reduction of iron oxides by reaction with carbon monoxide at high temperature (FeO + CO -> Fe + CO2). This reaction occurs in solid state, without fusion of the metal;
2. Cause the separation of impurities by forming liquid slag that can flow and separate from the metal.
This operation is successfully completed in a furnace fueled with charcoal. The slag formed is rejected and accumulates to form a heap near the work place (fig. 2).

Fig.3 Inside the tank of a great reduction furnace at Saré-Ma. Several big tuyeres are still inside the thank. Photo C. Robion-Brunner
Reduction furnaces
Reduction furnaces in the Dogon Country belong to the same family using natural ventilation (i.e. without bellows). They are all made above the ground over a pit dug into the soil. The walls are pierced with openings into which clay tuyeres are placed to allow air to infiltrate to the center of the tank (fig. 3). By contrast, the furnaces are of varying forms and size (from 1 to 4 m3), built with different materials (clay, bricks, stones, slag blocks, etc). In some furnaces, the melted slag flows laterally to the exterior (melt slag furnaces) and in others, the slag accumulates at the bottom of the furnace and forms large blocks (trapped slag furnaces).
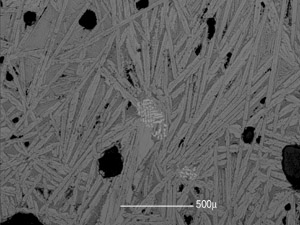
Fig.4 BSE image of a reduction slag. Fayalite : great middle grey crystals ; Wustite : dendrites and white globular grains ; Glass : dark grex background.Site of Fiko. Photo S. Perret
Reduction slag
Reduction slag is essentially formed of iron silicates (Fayalite – Fe2SiO4) with a variable proportion of grains of iron oxides (Wustite – FeO) and a vitreous phase (fig. 4). The forms vary and are essentially distinguished as:
1) melted slag, cooled outside the furnace, in the form of cords or a pile of cords;
2) internal slag, cooled inside the furnace in the form of a block.
The iron forge
The raw product of the operation is a block of slightly compacted metal still containing slag inclusions. The smith must compact and purify this raw metal before shaping it by plastic deformation while it is still hot. The metal is reheated in the hearth of the forge and hammered on an anvil (fig. 5). If necessary, the smith solders and applies thermic treatments (soaking, reheating, tempering) that improves the properties of the metal (hardness). Finally, the metal pieces are finished and mounted on a wood handle.
Forge slag
The impurities contained in the raw iron and the other substances present in the forge hearth (dust, ashes) or introduced by the smith (fluxes), as well as iron oxides that form on the surface of the metal, accumulate in the hearth. These materials are more or less melted and agglomerate to form roughly hemispheric slag lumps (fig. 6). This slag is also made of iron silicates (Fayalite – Fe2SiO4), but with a higher proportion of iron oxides (Wustite – FeO and Magnetite – Fe3O4).



